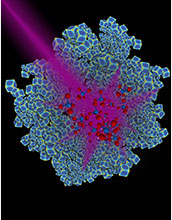
Methane is the building block of a lot of different fuels. There are a variety of methanes sources (think cows!) but on source of methane is to make it from carbon dioxide. There is lots of carbon dioxide but converting it to methane requires energy. Scientists at Duke University have developed a process that uses ultraviolet light and nanocubes made out of rhodium. Rhodium is an element that is pretty rare and the naocubes are about 37 nanometers on a side. When the UV light shines on the rhodium nanocubes the energy from the light helps to convert carbon dioxide into methane. The reaction is pretty specific and there aren’t a lot of other products besides methane. One byproduct they are trying to avoid is carbon monoxide. Taking carbon dioxide out of the atmosphere while making fuel is a neat way to help reduce global warming and also provide a renewable energy source.
This work was supported by the National Science Foundation along with Army Research Office and the Department of Energy.