Seth Darling is a scientist at the Argonne National Laboratory, a large national laboratory outside of Chicago. He is a materials chemist, and much of his work relates to nanoscale processes related to solar energy.
When you were a kid, what interested you about science?
I wanted to be a scientist for about as far back as I can remember. Chemistry was my first area of curiosity. I think it was probably a fascination with the ability to turn things into other things, and maybe a bit of learning how people blow stuff up.
When you were looking at careers, what attracted you to join a national research laboratory?
National laboratories like Argonne are amazing places because they sit at the
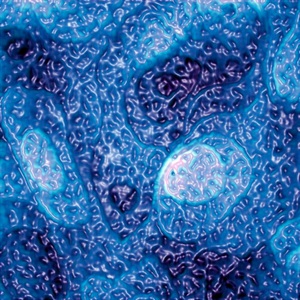
© Seth B. Darling, Argonne National Laboratory, Steven J. Sibener, University of Chicago; Making Waves Seth’s prize-winning nanoscale image, titled Rough Waters, was created in a lab with an atomic force microscope.
boundary between university research labs, which are primarily focused on basic science, and industrial research labs, which are primarily focused on developing products. That allows us to span the entire range of research, including translating new ideas into innovative technologies.
Working at a Department of Energy lab is perfect for me because my work is mostly on solar energy, and having an environment full of multidisciplinary experts all working on a big problem like the energy challenge is exciting.
What are your days like at work? What kind of science do you do?
No two days are alike. I spend some time in the lab performing experiments. I also write and review papers, work on proposals for research funding, present my work to other scientists, do outreach with schools and museums, and my favorite part: planning, strategizing about, and discussing science.
My research no longer fits into one area like chemistry or physics, which is a common situation for nanoscientists. Now my group works in a mixture of those fields plus materials science, electrical engineering, and even a little bit of economics. Our focus is on solar energy, but we also study topics like self-assembly (materials that organize themselves, like in the Rough Waters image) and nanoscale patterning.
You won first place in the National Science Foundation Visualization Challenge with the picture titled Rough Waters. Did you set out to take a neat picture or did it just happen?
We collected data that are the basis of this image as part of an experiment. We had no intention to capture something beautiful. The structure that the molecular layer exhibited was pretty cool-looking, so we did a bit of work to give the image a nice color and look, and this is the result.
You used a special instrument called an atomic force microscope. How does that work?
An atomic force microscope (AFM) is basically a super fancy record player. Record players are those things DJs use to scratch. It works in a completely different way than optical microscopes, and it is usually used to “see” things that are too small to see with visible light. It works by moving a very sharp tip back and forth across a surface and feeling the structures on the surface. A laser bounced off the back of the tip is used to measure the tiny movements. AFMs can even see individual molecules if the tip is sharp enough and there aren’t too many vibrations.
Where do all the shapes come from?
In this image, there are two types of shapes. There are larger rounded shapes and smaller dimples within these. The larger shapes are actually different layers of atoms of the gold surface that sits below the layer of molecules. The little dimples are a result of the fact that there are two types of molecules on the surface, and they have different heights. This difference in height is very small, less than a billionth of a meter, but an AFM can measure it quite accurately.
Do you think that you might become an artist and give up the life of a scientist?
Definitely no plans to stop being a scientist. I love my job. Also, I’d never cut it as an artist. Luckily, as a scientist you often get to use some artistic skills when preparing figures to communicate your results and ideas, so we can all dabble a bit.