Clean energy is a good thing. We need energy to power a lot of things around us (like cars and iPhones) but we also don’t want to harm the environment by putting things like carbon monoxide into the atmosphere. A number of energy producing devices use catalysts to help carry out chemical reactions. Fuel cells for example take chemical energy and convert it into electrical energy. Scientists at the University of New Mexico and 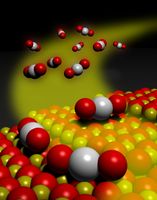 Washington State University have collaborated to develop a new kind of platinum catalyst to convert carbon monooxide (that invisible gas that can kill you) into carbon dioxide. Platinum is an expensive metal but also a very good catalyst. These scientists created a nanometer-scale material that traps platinum better and makes it a more efficient catalyst. The key was using something called cerium oxide to trap the platinum and keep it from forming aggregates which wasn’t good for catalysis. They hope to take these research discoveries and translate them into practical processes for making cleaner energy.
Washington State University have collaborated to develop a new kind of platinum catalyst to convert carbon monooxide (that invisible gas that can kill you) into carbon dioxide. Platinum is an expensive metal but also a very good catalyst. These scientists created a nanometer-scale material that traps platinum better and makes it a more efficient catalyst. The key was using something called cerium oxide to trap the platinum and keep it from forming aggregates which wasn’t good for catalysis. They hope to take these research discoveries and translate them into practical processes for making cleaner energy.
